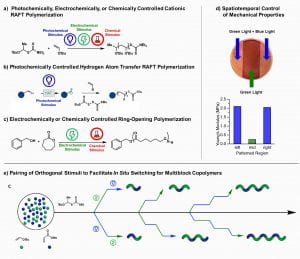
Externally Controlled Polymerizations
Synthesizing advanced polymeric materials with well-defined, complex structures is a grand challenge in polymer chemistry. In the Fors group, research focuses on accessing such materials through the utilization of external stimuli (e.g. light, electricity, or chemical additives) to gain spatiotemporal control over polymer initiation and termination. We have developed a range of externally controlled polymerizations, including photochemically/electrochemically/chemically controlled cationic RAFT, photochemically controlled hydrogen atom transfer radical RAFT, and electrochemically/chemically controlled ring-opening polymerizations. Notably, we have leveraged the spatiotemporal control and monomer selectivities offered by these methods to synthesize advanced materials, such as crosslinked polymer thin films with tunable mechanical properties or higher order multiblock copolymers, simply by controlling the stimulus applied to the reaction. Ongoing research in our group works to develop new synthetic and catalytic strategies for externally controlled polymerizations and to extend our research to more complex structures and architecture.
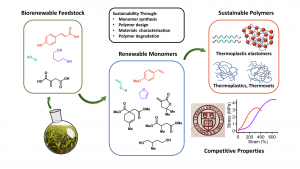
Sustainable Polymers
Commercial polymers serve many vital functions which have allowed our society to reduce disease, food waste, transportation emissions, and production costs. To reduce the impact that polymer production has on our environment, our group has developed several sustainable pathways to monomers from biorenewable feedstocks. Utilizing radical, cationic, and polycondensation polymerizations, we have produced biorenewable thermoplastics, thermosets, and thermoplastic elastomers. Most recently, we have leveraged the controlled cationic polymerization methods developed in our lab to produce strong thermoplastics and thermoplastic elastomers that can be sourced entirely from bioalcohols. Ongoing research focuses on the synthesis of new renewable monomers, development of degradable/depolymerizable polymers, upcycling of commodity plastics, design and characterization of robust materials, and adoption of green chemistry methods. As members of the NSF Center for Sustainable Polymers, we also cherish our collaborations and interdisciplinary work within the sustainability community.
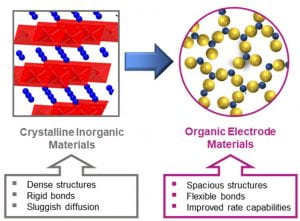
Energy Materials
Batteries with fast-charging capabilities are needed to meet the growing energy demands of modern technologies. Counter ion transport in inorganic materials currently limits charge/discharge rates in lithium-ion batteries, however, weak intermolecular forces in organic materials result in flexible, spacious structures that offer improved ion transport capabilities. By exploiting these properties, our group – in collaboration with the Abruña group – has been able to synthesize polymeric battery materials that reach over 90% charge in under a minute. We wish to understand structure-property relationships in battery materials and use this to inform future high-performance organic batteries.
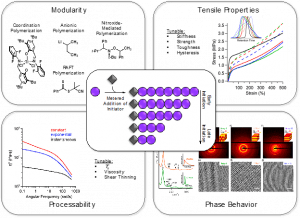
Controlling the Shape of the Molecular Weight Distribution
Three terms are typically used to describe the molecular weight distribution (MWD) of a polymer: the number average molecular weight (Mn), the weight average molecular weight (MW), and the dispersity (Ð), which describes the relative breadth of the distribution. While the impact of each of these terms on polymer properties has been extensively studied, the influence of the shape of the MWD has been underexplored. This has largely been due to a lack of synthetic methods that provide absolute control over the polymerization. To address this challenge, our group has developed a versatile and modular strategy for temporally controlling the rate of initiation in living polymerizations, resulting in predictably and precisely tailored MWD shapes. Our research has demonstrated that variations in MWD shape have profound influence over a wide variety of polymer properties, from material properties like viscosity and tensile strength to block copolymer phase behavior. Ongoing research in the Fors group aims to expand upon our understanding of the relationship between MWD shape and polymer properties, as well as to apply our synthetic strategy toward other polymerization systems.